Researchers at Oak Ridge National Laboratory (ORNL) and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.
Results published in the Journal of the American Chemical Society provide new insights on the way molecules pack and order themselves into “tunable” interfaces, monolayer thick surfaces with structures that can be modified for specific functionalities.
“Understanding the design rules of the chemistry happening at the liquid-liquid interface ultimately informs how we can make new materials with custom properties,” said Benjamin Doughty of ORNL’s Chemical Sciences Division.
The studied oligomer is an amphiphilic molecule, meaning parts of its structure are hydrophobic while others are hydrophilic. When samples stabilized in oil are introduced into a water-based solution, the molecules self-assemble in response to their mixed attraction and repulsion to water.
“Being able to observe in real time how these molecules arrange at a varied interface is a broadly applicable fundamental scientific accomplishment,” Doughty said.
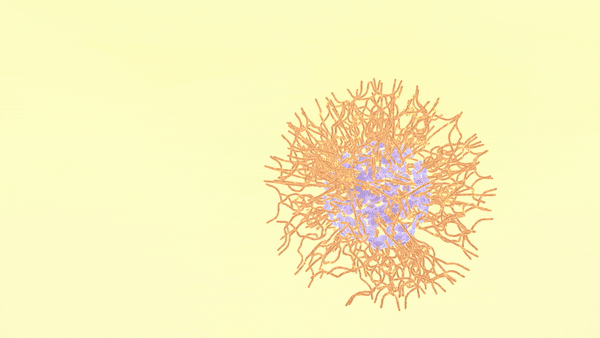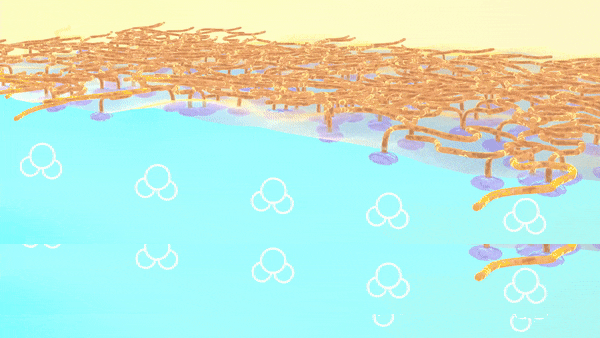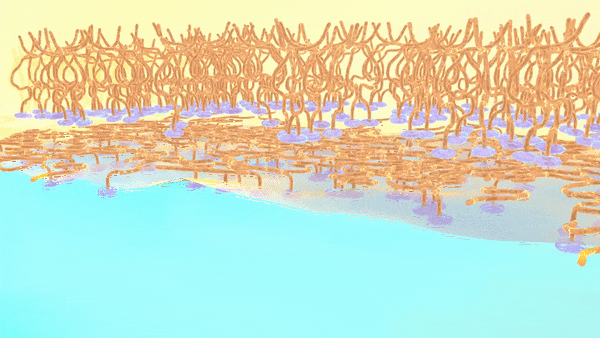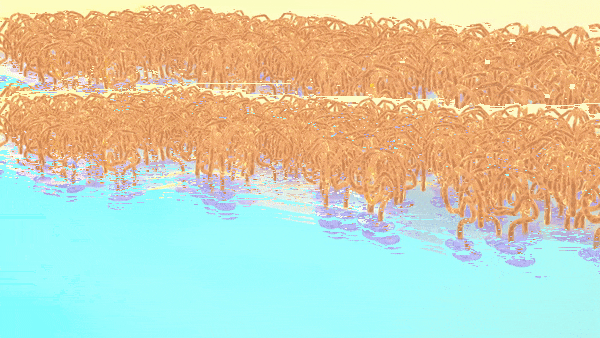
As shown in the animation, the charged oligomer heads home in on the water phase; but the flexible tails coil up in the oil when they have room to spare, or tighten to accommodate neighbors as the interface becomes crowded.
“We discovered that adjusting the ions, or charged particles, in the water phase aided in the formation of well-defined interfaces, with oligomers taking on more tightly coiled structures,” Doughty said.
Too few ions and the tails spread out loosely, leaving gaps; too many, and they squeeze in, ballooning from the surface.
Tailoring surfaces on a molecular level to design new materials opens possibilities not only for biocomputing but also broadly for chemical separations, sensing, and detection.
“Observing the liquid-liquid interface helps us understand the chemistry that drives all of these technologies,” said Doughty.
The journal article is published as Insight into the Mechanisms Driving the Self-Assembly of Functional Interfaces: Moving from Lipids to Charged Amphiphilic Oligomers.
Click here to read more about the research on ORNL website.