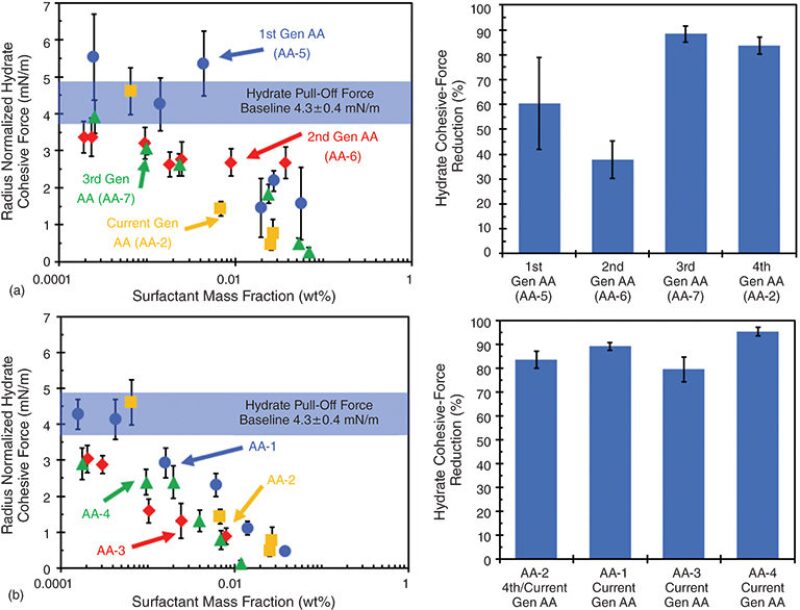
A common hydrate-management strategy involves the use of large volumes of thermodynamic inhibitors (THIs) to operate outside the hydrate-stability region, However, this strategy represents significant capital expenditure and operating costs. Low-dosage hydrate inhibitors (LDHIs), in the form of kinetic hydrate inhibitors (KHIs) and antiagglomerants (AAs), present an economical alternative to THIs. In this study, a quantitative micromechanical force (MMF) has been deployed to study the performance of seven industry AAs. The results illustrate that an effective AA is one that lowers the cohesive forces between hydrate particles.
Introduction
AAs prevent hydrate agglomeration of a steady-state hydrate slurry.
×


Continue Reading with SPE Membership
SPE Members: Please sign in at the top of the page for access to this member-exclusive content. If you are not a member and you find JPT content valuable, we encourage you to become a part of the SPE member community to gain full access.